how to prepare saturated salt solution Saturated salt solution
Hey there! I stumbled upon some interesting information about the chemistry of solutions and I couldn’t wait to share it with you. So, let’s dive right in!
Imagine yourself in a chemistry lab, conducting an exciting experiment. You’re about to create a saturated NaCl solution. The first step is to add 15 grams of sodium chloride to a container filled with water. The image below perfectly illustrates this process:
Create a Saturated NaCl Solution
 Image source: water.mecc.edu
Image source: water.mecc.edu
Now that you’ve successfully prepared a saturated NaCl solution, let’s dig deeper into the chemistry behind it. Understanding the properties of solutions is crucial in various scientific fields.
In chemistry, a solution refers to a homogeneous mixture composed of two or more substances. In this case, our NaCl solution consists of sodium chloride (NaCl) dissolved in water. This process, known as dissolution, is essential for creating a solution with evenly dispersed particles. It’s fascinating to witness how different substances interact with each other on a molecular level.
But, what happens to the sodium chloride molecules when dissolved in water? Well, the NaCl crystals disintegrate into individual sodium (Na+) and chloride (Cl-) ions. These ions disperse and freely move within the water, creating a solution with a balanced concentration of solute (NaCl) and solvent (water) molecules.
Now that we’ve explored the chemistry side of things, let’s take a closer look at the visual representation of a saturated NaCl solution:
Visual Representation of a Saturated NaCl Solution

As you can see, the image beautifully depicts the arrangement of sodium and chloride ions within the solution. The dissolved ions are evenly dispersed, ensuring a saturated solution.
Understanding the chemistry of solutions is essential for a wide range of applications. From understanding the properties of different liquids to creating pharmaceuticals, solutions play a crucial role in our everyday lives.
So, the next time you come across a sodium chloride solution, you’ll know exactly how it’s made and the fascinating science happening behind the scenes!
Remember, knowledge is power!
If you are looking for What is an unsaturated solution? you’ve visit to the right web. We have 5 Pictures about What is an unsaturated solution? like What is an unsaturated solution?, What is a Saturated Solution - Preparation, Types & Examples and also Lesson 8: The Chemistry of Solutions. Read more:
What Is An Unsaturated Solution?
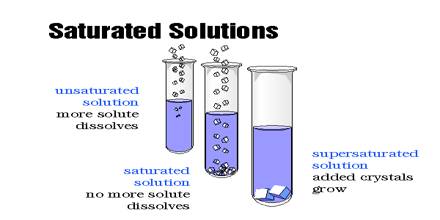 mitopencourseware.web.fc2.comSolved Prepare A Saturated NaCl Solution By Adding 15 G Of | Chegg.com
mitopencourseware.web.fc2.comSolved Prepare A Saturated NaCl Solution By Adding 15 G Of | Chegg.com

Saturated Salt Solution - Stock Image - C043/0515 - Science Photo Library
 www.sciencephoto.comsaturated
www.sciencephoto.comsaturated
What Is A Saturated Solution - Preparation, Types & Examples
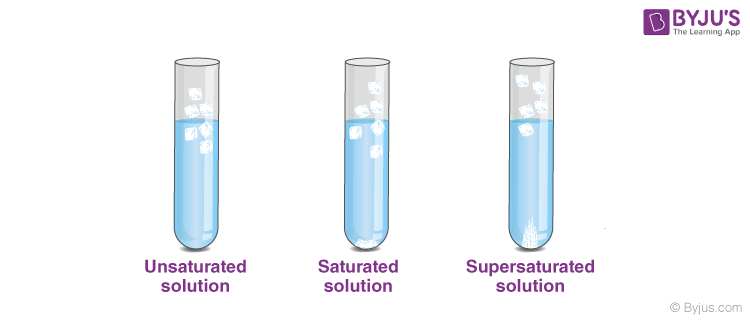 byjus.comsaturated unsaturated saturation preparation byjus examples substances mixture
byjus.comsaturated unsaturated saturation preparation byjus examples substances mixture
Lesson 8: The Chemistry Of Solutions
 water.mecc.eduSaturated salt solution. Saturated unsaturated saturation preparation byjus examples substances mixture. Solved prepare a saturated nacl solution by adding 15 g of
water.mecc.eduSaturated salt solution. Saturated unsaturated saturation preparation byjus examples substances mixture. Solved prepare a saturated nacl solution by adding 15 g of